Master Formulation Record Overview
This topic is for independent pharmacies only.
The Master Formulation Record for a non-sterile preparation includes all necessary information to compound the mixture.
This information can be entered directly into Propel Rx in the Mixture Master Formula window. It can be accessed from the Mixture Folder by selecting the Master Formula button. The information prints on the supplementary label for a prescription filled for the mixture, Mixture Breakdown report, and mixture recipe report.
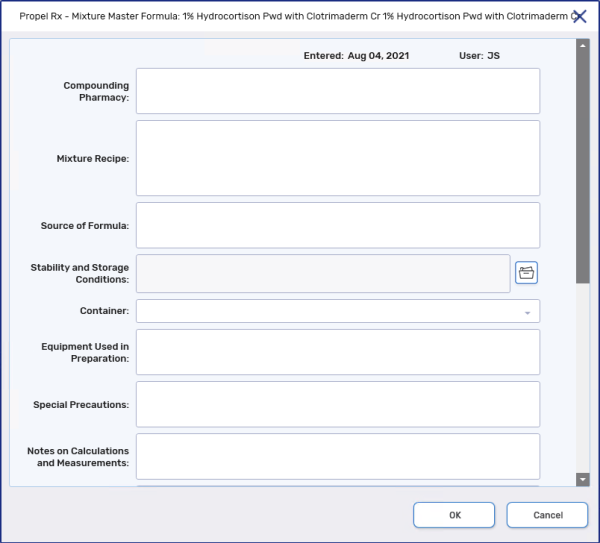
Use the dropdown below to learn more information about each of the field.
-
Entered - the date the Master Formulation Record was created or last updated.
-
User - the user that created or last updated the Master Formulation Record.
-
Compounding Pharmacy - the pharmacy that compounded the mixture.
-
Mixture Recipe - the mixing instructions, which may include:
-
order of mixing
-
mixing temperatures
-
duration of mixing
-
-
Source of Formula - the source or origin of the formula.
-
Stability and Storage Conditions - a selection of storage conditions for the mixture.
You can select multiple storage conditions by holding the CTRL key on your keyboard while making your selections.
You can add custom storage conditions in List Maintenance. For more information, see Mixture Storage in List Maintenance.
-
Container - use the folder icon
 to select the container for the mixture.
to select the container for the mixture.You can add custom containers in List Maintenance. For more information, see Containers in List Maintenance.
-
Equipment Used in Preparation - materials and equipment required to create the mixture.
-
Special Precautions - any special precautions to be observed by compounding personnel.
-
Notes on Calculations and Measurements - any calculations, measurements or ingredient preparation for the mixture.
-
Description of Final Product
-
Quality Controls - quality control procedures and expected results, including how the lot number was created.
-
Training - any specialized training that pharmacy staff must undergo before creating the mixture.
-
References and Notes - documentation supporting the preparation of the mixture.
-
Disposal Instructions for Patients
-
Miscellaneous
-
Additional Instruction Label - additional information about the ingredients required for compounding. This information only appears on the Supplementary label and not on the Mixture Breakdown report or recipe.